Statistical Quality Analysis
This course represents the basis for Statistical Process Control (SPC). All the three main analysis typically used in Quality Statistics are covered by the program of this course: Measurement System Analysis, Control Charts, Capability Analysis. These statistical tools are often misunderstood and misused, leading to false alarms or, even worst, to the impossibility of detecting an actual problem in the process. The course will take you among the experts of these topics!
Topics include
- Introduction to Quality Statistics
- Definition of industrial quality
- The curse of variability
- Introduction to Measurement System Analysis
- Precision and accuracy
- Repeatability and reproducibility
- Gage R&R analysis
- Attribute agreement analysis
- Gage linearity and bias
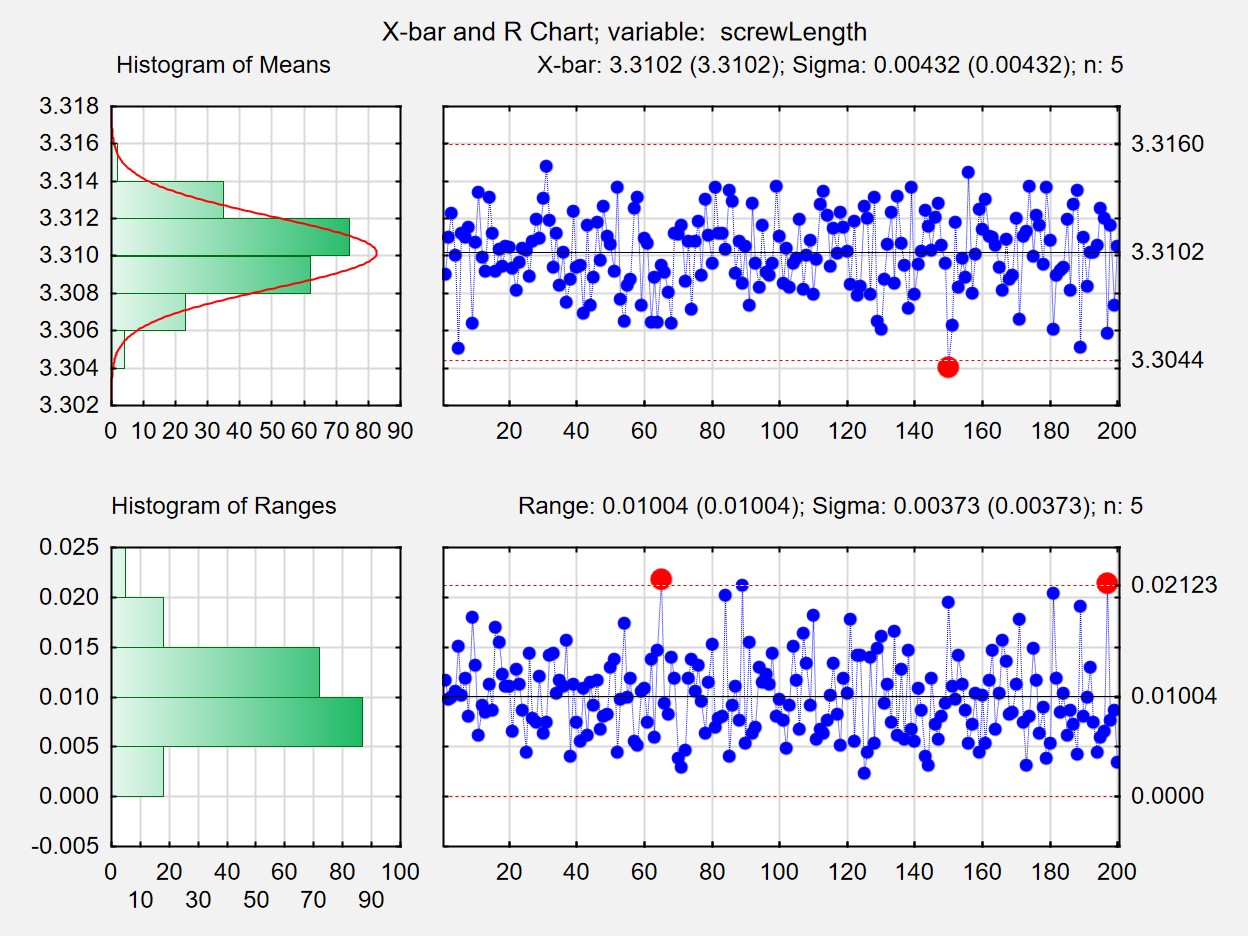
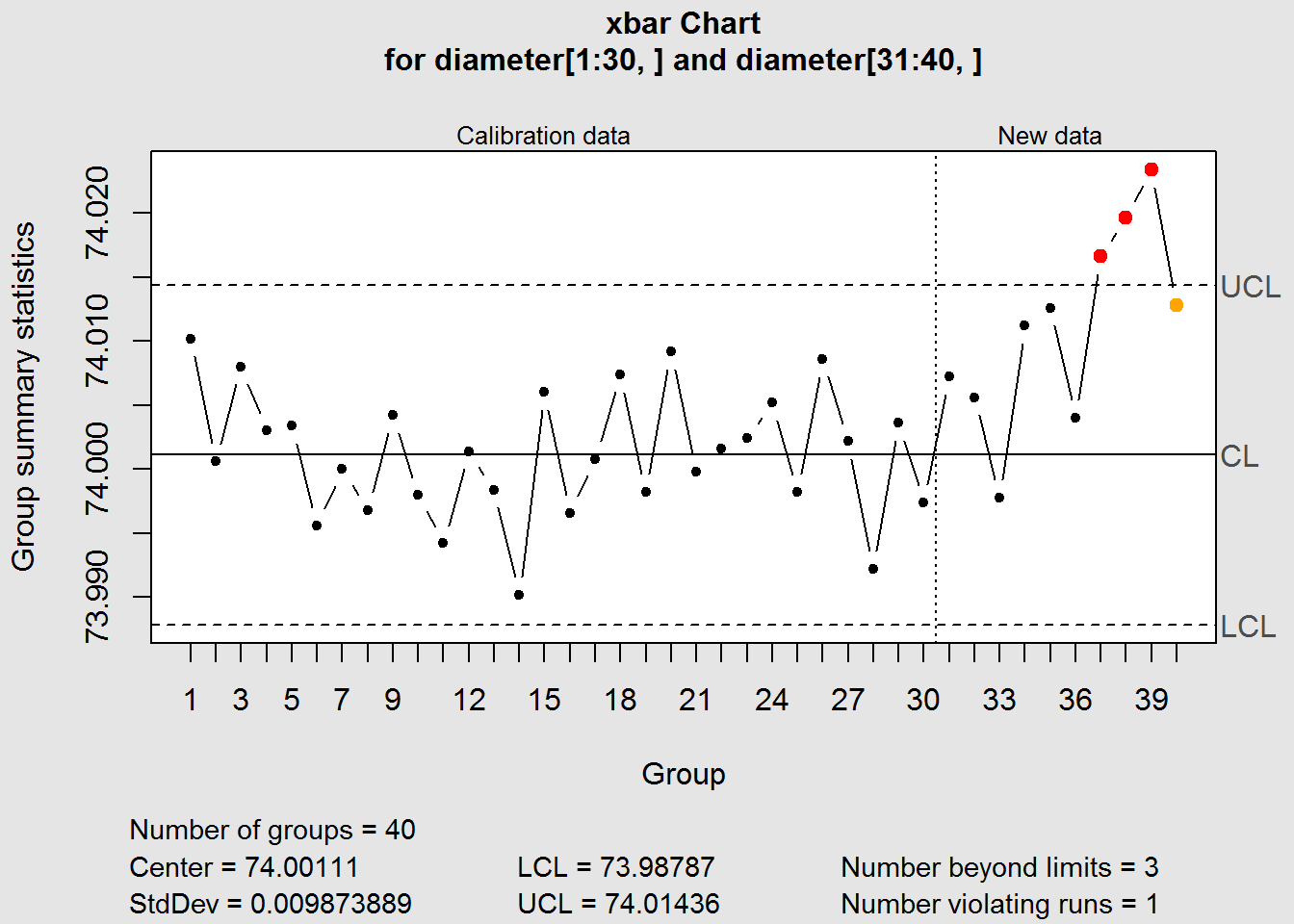
- Introduction to control charts
- Definition of an under control process
- X-bar, R and S charts
- Control charts for individual measures
- Moving range chart
- Control charts for attribute data
- Definition and control of defectives and defects
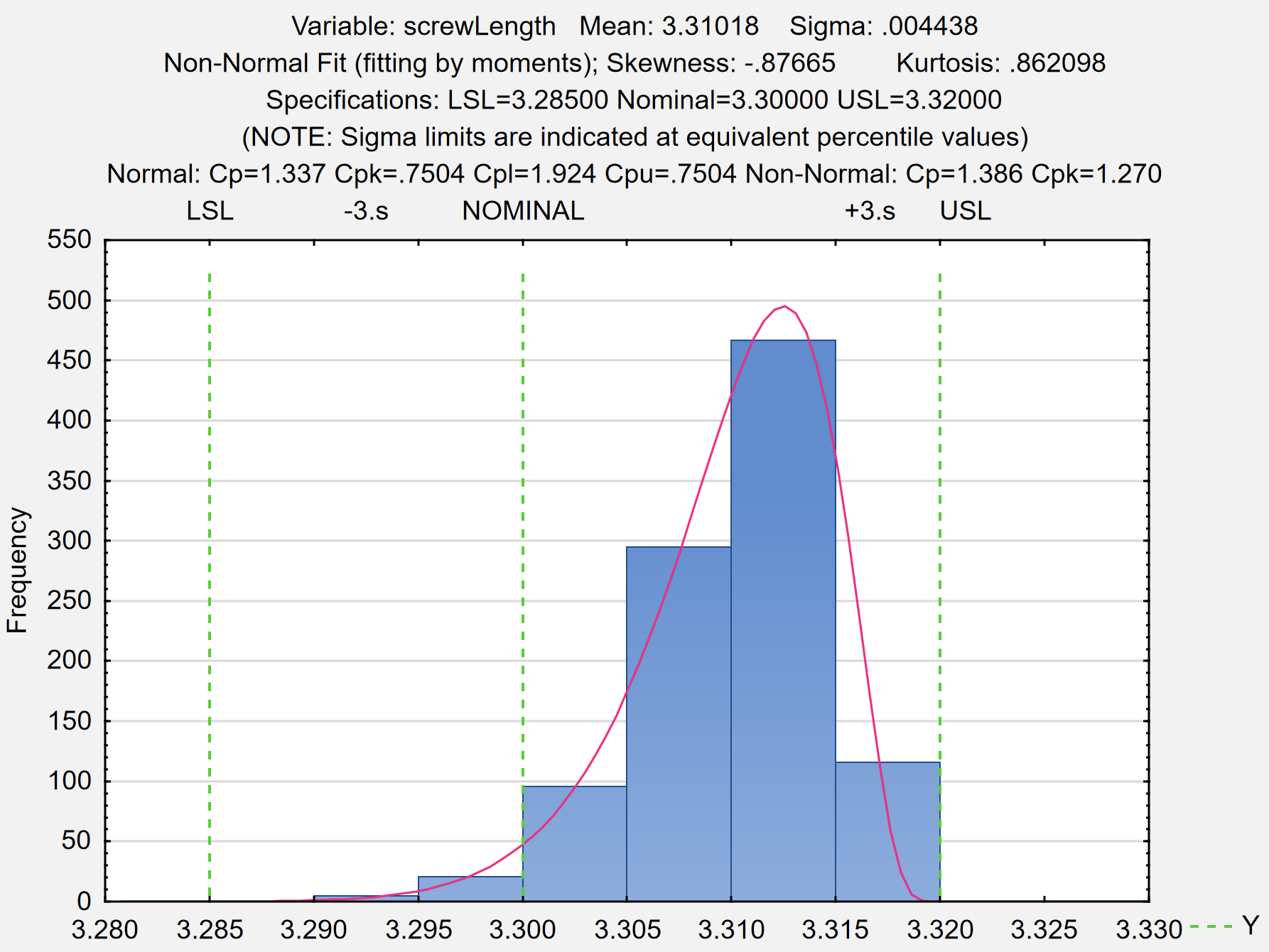
- Introduction to capability analysis
- Capability analysis for normal data
- Understanding and using capability indexes: Ppk, Cpk, Cpm, Pp and Cp
- Capability for non normal data
- Finding a good distribution to fit data
- Common errors and mistakes with control charts and capability
- Differences between control limits and specification limits
- Using Monte Carlo simulation to improve capability
- Relation between DOE and capability analysis
- Introduction to Data Science approaches for predictive control charts
- Even further: a glance on predictive maintenance
What you will be able to do
- Understand if measured values are reliable
- Test operators ability to collect good data
- Monitor a process and understand if something unusual is happening
- Understand if the process is working according to specifications
- Suggest how to optimise an unstable or low capable process
Duration
1 day.
Pre requisites
Basic Statistics.
Available based on
- TIBCO Statistica
- Minitab
- R
- Python
Audience
This course is designed for quality professionals and is fully covering every statistical aspect required both in manufacturing in general and in pharmaceuticals.